Evgen Pharma’s lead candidate SFX-01 has been selected for evaluation in a randomised Phase II/III trial, to be sponsored by the University of Dundee, which aims to investigate its potential as a treatment for COVID-19.
Specifically, the trial will look at whether SFX-01 can reduce the severity, or prevent the onset of, acute respiratory distress syndrome (ARDS) associated with COVID-19, and thus reduce the need for invasive patient ventilation.
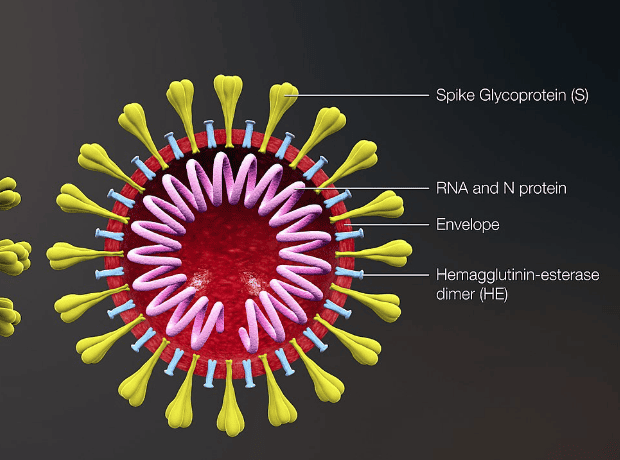
SFX-01 upregulates the Nrf2 pathway which is part of the natural human defence against inflammatory and oxidative stress, such as the inflammation that occurs during a severe viral infection.
According to the firm, preclinical evidence shows that up-regulating the Nrf2 pathway can reduce the severity of ARDS, the progressive lung damage observed in COVID-19 patients which can lead to the need for invasive ventilation.
The trial, which is expected to begin enrolment in July, is being led by Professor James Chalmers, British Lung Foundation Professor of Respiratory Research at the University of Dundee, with support from a LifeArc grant.
It will recruit up to 300 patients with confirmed or suspected COVID-19 from hospitals across the UK; half will receive SFX-01 in addition to standard hospital care while the other half will receive a placebo and standard hospital care.
“SFX-01 is an anti-inflammatory medication that we believe may have the potential to reduce some of the worst outcomes of COVID-19. Early treatment with an Nrf2 activator in patients hospitalised with COVID-19 may prevent deterioration and help to preserve precious ICU resources in the context of the pandemic. This is a completely new mechanism as there is currently no drug that targets Nrf2,” said Professor James Chalmers, principal investigator on the trial.