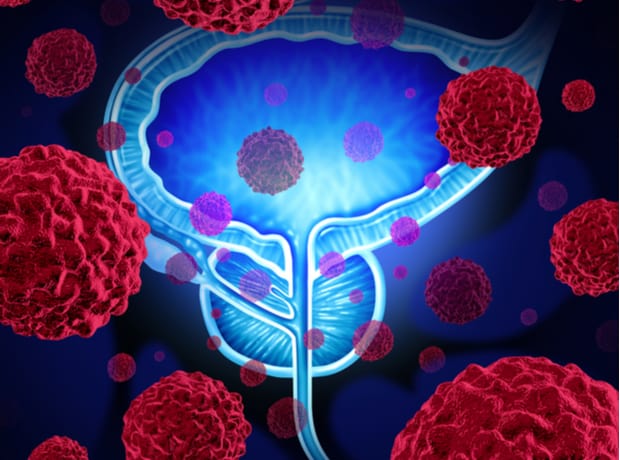
The National Institute for Health and Care Excellence (NICE) has published draft guidance rejecting Janssen’s Erleada (apalutamide) with androgen deprivation therapy (ADT) for treating prostate cancer.
The draft guidance considers Erleada for treating hormone-relapsed prostate cancer in adults who are at high risk of the cancer spreading, or hormone-sensitive prostate cancer that has already metastasised.
Erleada is an androgen receptor inhibitor which works by blocking the effect of testosterone on prostate cancer cells.
Current treatment for hormone-relapsed non-metastatic prostate cancer is ADT, alone or with darolutamide (another androgen receptor inhibitor). Current treatment for hormone-sensitive metastatic prostate cancer is docetaxel (a chemotherapy drug) plus ADT or ADT alone for people who cannot take docetaxel.
Trial results suggest that, compared with placebo plus ADT, Erleada plus ADT improves both progression-free survival and survival. However, NICE considers the evidence is uncertain because in the trial some people could switch to other treatments, and some could receive treatments not available in the NHS.
Also, estimates of how long it took for disease to worsen after the clinical trial had ended and how long people live are uncertain, the Institute noted.
Therefore the cost-effectiveness estimates in the analyses – which best reflect the committee’s preferred assumptions – have fallen above what NICE considers an acceptable use of NHS resources, and so it was unable to recommend funding for the drug in this setting.